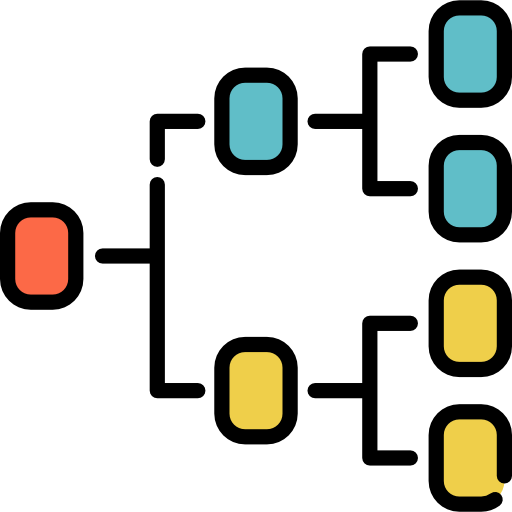
The Orphanet nomenclature is a multilingual, standardised, controlled medical terminology specific to rare diseases, that includes all clinical entities registered in the Orphanet knowledge base. Each clinical entity (disorder, group of disorders, or subtype of a disorder) is associated with a unique numerical identifier named ORPHAcode, as well as a preferred term, synonyms, and a definition. The Orphanet nomenclature is organised in a classification system, structured around the major medical specialties, according to diagnostic and therapeutic relevance. This clinical coding system is mapped to the main generic clinical and genetic terminologies, thus providing a common language across healthcare and research systems for effective monitoring and reporting on all rare diseases diagnosis, ultimately improving their visibility.
The Orphanet Nomenclature of rare diseases is produced according to standard procedures and updated based on the literature and collaborations with expert groups, so as to reflect the evolution of knowledge.
ORPHAcodes files are available via the CC BY 4.0 licence and are released annually* in July.
The Orphanet nomenclature pack compiles various files which provide the computable information necessary to achieve implementation of ORPHAcodes in health information systems, and ensure accurate coding. Differentials are provided to ensure traceability*.
The ORPHAcodes API facilitates the informatic access to the nomenclature pack data and allows flexible implementation into the various IT systems in use in the different countries and/or settings.
You have a question related to the Orphanet nomenclature content and the implementation of ORPHAcodes in Health Information Systems, contact us via GitHub
a tool dedicated to browse the Orphanet classifications; it allows searching for clinical entities by ORPHAcode.
a tool that facilitates transcoding by allowing users to search for rare clinical entities and displaying the corresponding mappings in aligned generic medical and genetic terminologies.
a tool that allows visualisation of scientific data and classification information associated to rare diseases in a user-friendly format.
see here other Orphanet standard procedures.
The Orphanet nomenclature of RD is included in the 2011 Cross Border Health-care Directive as far as RD diagnosis is concerned. In 2014, the European Commission Expert Group on Rare Diseases also promoted the inclusion of ORPHAcodes in health information systems. ORPHAcodes were recognised as a best practice in 2017 by the EC’s Steering Group on Promotion of health and Prevention of non-communicable diseases (SGPP).
ORPHAcodes are listed in the set of common data elements for Rare Diseases Registration released by the EU RD platform to ensure interoperability between registries.
ORPHAcodes are the recommended code to trace RD diagnoses in the European Common Semantic Strategy 2019 & in the Guidelines on Patient Summary, Release 3.2, Mar 2022 & Release 3.4, November 2024.
The functional specifications for the EC’s eHealth Network guidelines on the European Electronic Health Records format ( EEHRxF) include the ORPHAcodes for inclusion in the Patient Summary as such ORPHAcodes (aggregation level-disorder level and subtypes) are included in the Code Systems used in the MVC – My Health @ EU – eHealth Digital Service Infrastructure (eHDSI).
The WHA Resolution “ Rare Diseases: a Global Health Priority for Equity and Inclusion”, adopted on 24 May 2025, urges Members States to commit to considering the implementation of ICD-11, and where appropriate, interoperable codification systems for rare diseases such as the Orphanet nomenclature of rare diseases, at their earliest possibility, and in accordance with their available resources, in order to enable the recording, reporting and monitoring of rare diseases at the national and international levels.
This nomenclature and classification system has been developed and maintained thanks to European support (RD-PORTAL S12305098-2006119; RD-PORTAL2 S12324970-20091215; ORPHANET EUROPE JOINT ACTION 20102206 ; EUCERD Joint Action 2011 22 01; ORPHANET OPERATING GRANT 20133305; RD-ACTION JOINT ACTION 677024; ORPHANETWORK DIRECT GRANT 831390; RD-CODE 826607; OD4RD 101070531; OD4RD2 101110100) since the recognition as a priority, in the Council Recommendation of June 8th 2009 on an action in the field of RD, of the improvement in codification of RD.